Vitamin D deficiency and hair loss
Vitamin D plays an important role in calcium homeostasis and bone health. It has been demonstrated that vitamin D is also associated with several autoimmune diseases such as lupus, type I diabetes mellitus, rheumatoid arthritis, multiple sclerosis, vitiligo, and psoriasis among others [Hewison, 2012]. Deficiencies in vitamin D may derive in abnormal autoimmunity responses [Arnson, 2007]. Furthermore, vitamin D and its receptor have been implicated in the pathogenesis of diverse forms of hair loss [Conic et al., 2018].
We obtain a small amount of vitamin D from our daily diet, however most of the vitamin D in our body is synthesized by keratinocytes in the epidermis after exposure to solar radiation. Vitamin D acts in human body cells through the vitamin D receptor (VDR), triggering a signaling cascade which modulates the transcription of target genes involved in regulating homeostasis. VDR is a nuclear receptor expressed in numerous cells and tissues in the human body including the skin. VDR is expressed in the two major cell populations conforming the hair follicle: epidermal keratinocytes and dermal papilla cells. Studies have demonstrated that absence of VDR leads to hair loss by hair cycle dysregulation [Mady et al., 2016].
The first evidence suggesting a role of VDR in hair cycle was derived from the observation of alopecia in patients with type IIA vitamin D-dependent rickets [Brooks et al., 1978]. Vitamin D-dependent rickets type IIA is a disorder in bone formation caused by a defect in the VDR gene. Interestingly most of the patients with this disorder also had alopecia. However, the molecular mechanisms by which vitamin D and its receptor VDR regulate hair cycle are still unknown.
Hair regeneration depends on the activation of hair follicle stem cells. The hair follicle is an organ that undergoes cyclic involution and regeneration throughout life [Paus et al., 1999]. The hair cycle consists in three cycling phases: anagen (growth), catagen (involution), and telogen (resting). In the telogen phase, the dermal papilla and the bulge come closer together and this approximation allows signaling between the dermal papilla and keratinocyte stem cells in the bulge, inducing a new proliferative anagen phase [Paus et al., 1999] as observed in the next Figure.
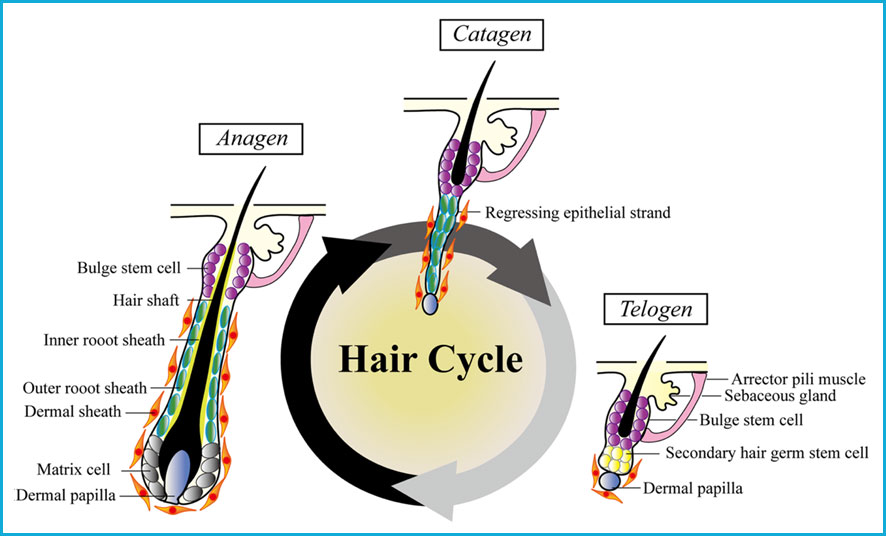
The expression of VDR is increased in the hair follicle during the late anagen and catagen phases of the hair cycle; correlating with decreased proliferation and increased differentiation of keratinocyte stem cells [Demay et al., 2007]. VDR is essential for the start of a new anagen phase. Several mutations have been found which may interfere with VDR’s function increasing the risk of hair loss [Simon et al., 2010].
Additionally, levels of serum vitamin D have also been correlated to hair loss. Diverse studies have demonstrated insufficiency or significantly lower serum levels of vitamin D in patients with Alopecia Areata as compared to healthy controls [Yilmaz et al., 2012; Aksu et al., 2014; Ghafoor et al., 2017; Gade et al., 2018]. Supplementation with vitamin D has also been observed to protect hair follicles from chemotherapy-induced alopecia [Wang et al., 2006; Jimenez et al., 1996].
References:
Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf). 2012;76:315–25.
Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–42.
Conic, R., Piliang, M., Bergfeld, W., & Atanaskova-Mesinkovska, N. (2018). Vitamin D Status in Scarring and Non-Scarring Alopecia. Journal of the American Academy of Dermatology, S0190-9622(18)30631-5.
Mady, L. J., Ajibade, D. V., Hsaio, C., Teichert, A., Fong, C., Wang, Y., Christakos, S., & Bikle, D. D. (2016). The Transient Role for Calcium and Vitamin D during the Developmental Hair Follicle Cycle. The Journal of investigative dermatology, 136(7), 1337–1345.
Brooks, M. H., Bell, N. H., Love, L., Stern, P. H., Orfei, E., Queener, S. F., Hamstra, A. J., & DeLuca, H. F. (1978). Vitamin-D-dependent rickets type II. Resistance of target organs to 1,25-dihydroxyvitamin D. The New England journal of medicine, 298(18), 996–999.
Paus, R., & Cotsarelis, G. (1999). The biology of hair follicles. The New England journal of medicine, 341(7), 491–497.
Demay, M. B., MacDonald, P. N., Skorija, K., Dowd, D. R., Cianferotti, L., & Cox, M. (2007). Role of the vitamin D receptor in hair follicle biology. The Journal of steroid biochemistry and molecular biology, 103(3-5), 344–346.
Simon, K. C., Munger, K. L., Xing Yang, & Ascherio, A. (2010). Polymorphisms in vitamin D metabolism related genes and risk of multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England), 16(2), 133–138.
Yilmaz N, Serarslan G, Gokce C. Vitamin D concentrations are decreased in patients with alopecia areata. Vitam Miner. 2012;1:105–109.
Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299–1304.
Ghafoor R, Anwar MI. Vitamin D deficiency in alopecia areata. J Coll Physicians Surg Pak. 2017;27:200–202.
Gade VKV, Mony A, Munisamy M, Chandrashekar L, Rajappa M. An investigation of vitamin D status in alopecia areata. Clin Exp Med. 2018;18:577–584.
Wang J, Lu Z, Au JL. Protection against chemotherapy-induced alopecia. Pharm Res. 2006;23:2505–14.
Jimenez JJ, Yunis AA. Vitamin D3 and chemotherapy-induced alopecia. Nutrition. 1996;12:448–9.
Chen, C., Huang, W., Wang, E.H.C. et al. Functional complexity of hair follicle stem cell niche and therapeutic targeting of niche dysfunction for hair regeneration. J Biomed Sci 27, 43 (2020).

